Think you’re a climate change genius? Strap in and prepare for whiplash.
To close out last week’s post, I referenced a Forbes article, Green Hydrogen’s Hype Hits Some Very Expensive Hurdles, which seeded the roots for this week’s Energy Rant. It referenced a Cornell University paper claiming carbon capture from the manufacture of blue hydrogen “is energy intensive and leads to even more climate pollution than if CO2 just wafted into the air.”
Whoa, ho, Nellie! Does carbon capture result in more emissions and energy consumption than free release of combustion products? The latter isn’t surprising, but more emissions are a headliner. I had to investigate. Beelzebub is in the details.
Here’s the rub: it’s all based on an assumed “fugitive methane” (leak) rate of 3.5% for natural gas from well to the point of use per Cornell’s paper.
Blue Hydrogen and Reformulation
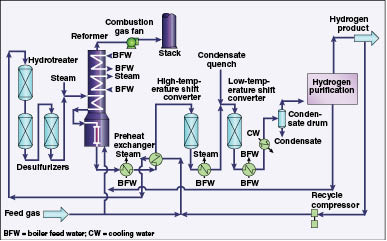
Blue hydrogen is gray hydrogen plus carbon capture. Gray hydrogen is produced by a mature process called steam-methane reforming. Methane (CH4, i.e., natural gas) is pressurized with steam at about 1,600F and 360 psi. In the presence of a catalyst, this high-pressure stew produces hydrogen (H2) and carbon monoxide (CO)[1]. The combustion of natural gas supplies heat.
![]() The carbon monoxide (CO) is used with water (H2O) in a second and third reaction in the presence of a different catalyst to produce a “water-gas shift” reaction to produce one more molecule of hydrogen[2].
The carbon monoxide (CO) is used with water (H2O) in a second and third reaction in the presence of a different catalyst to produce a “water-gas shift” reaction to produce one more molecule of hydrogen[2].
![]() Here is a cartoon of the process from Chemical Engineering Processing:
Here is a cartoon of the process from Chemical Engineering Processing:
Reader Challenge
According to Wikipedia, hydrogen reformulation from natural gas produces 5.45 tons of CO2 per ton of H2, not counting the heat source. Here is a challenge for readers: Show me where I made a mistake with the following analysis. You’ll be a star for a week.
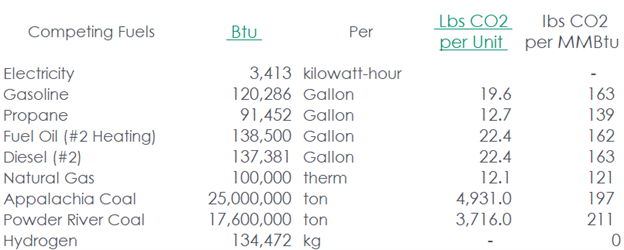
First, I calculate emissions from the reformulation in pounds of CO2 per million Btu of energy (higher heating value).
 Then, I calculated the emissions required to heat the process, which, according to Wikipedia (below), is 6.2 MWh thermal per 1.1 ton of hydrogen produced.
Then, I calculated the emissions required to heat the process, which, according to Wikipedia (below), is 6.2 MWh thermal per 1.1 ton of hydrogen produced.
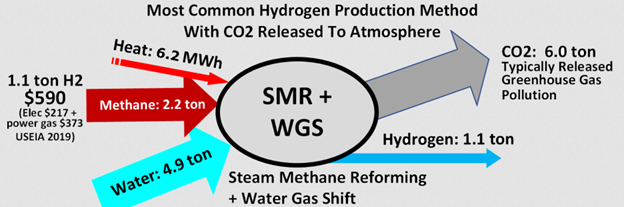
 I take the 89.2 lb/MMBtu from the process and add the 23.8 lb/MMBtu for the heat for a total of 113.8 lb/MMBtu. Burning natural gas straight produces 121 lb/MMBtu, again, a higher heating value. Getting higher emissions by burning natural gas for heat versus using natural gas as the feedstock and source of heat for hydrogen production is a second law reverser—not going to happen. I question the numbers in the Wikipedia cartoon. What’s wrong here?
I take the 89.2 lb/MMBtu from the process and add the 23.8 lb/MMBtu for the heat for a total of 113.8 lb/MMBtu. Burning natural gas straight produces 121 lb/MMBtu, again, a higher heating value. Getting higher emissions by burning natural gas for heat versus using natural gas as the feedstock and source of heat for hydrogen production is a second law reverser—not going to happen. I question the numbers in the Wikipedia cartoon. What’s wrong here?
Here are the source data.
An Order of Magnitude Fugitive Methane
Ok, methane released into the atmosphere is a potent greenhouse gas – 25 times more powerful than CO2 over a 100-year analysis period and 72 times more potent over a 20-year period. The difference is its longevity in the atmosphere. CO2 hangs around longer. I’ll come back to this. Fugitive (leaking) hydrogen is the mechanism for the claim that blue hydrogen, with carbon capture, per definition, might result in 20% more greenhouse gases (GHGs) “than burning natural gas or coal for heat and some 60% greater than burning diesel oil for heat, again with our default assumptions,” per the Cornell paper.
When I read those comparisons to other fuels, I thought they must be punishing natural gas and its renegade, er, fugitive losses. Sixty percent more emissions than burning diesel (fuel oil)? Fuel oil emits about a third more CO2 at the point of use than natural gas.
What about the claimed 3.5% renegade losses? As it turns out, estimates of apostate natural gas range from, oh, 0.5% to 8.0% – only an order of magnitude. Beelzebub.
 Physics of GHGs
Physics of GHGs
As I wrote eight years ago (wow, time flies), greenhouse gases comprise molecules with three or more atoms. E.g., CO2, H2O, CH4, and N2O, whip it good!
I took a vibrations course as an undergraduate, and let’s just say that’s why I ended up in the thermal track of mechanical engineering rather than the mechanical track. The study of vibrations includes degrees of freedom and resonance. Diatomic molecules, e.g., O2, N2, H2, have one vibration mode and no resonance. Larger molecules have 3N-5 or 3N-6 vibration modes for linear and non-linear molecules, respectively, where N is the number of atoms in the molecule. CO2 is linear and, therefore, has four modes of vibration. Methane, CH4, has nine modes of vibration, etc.
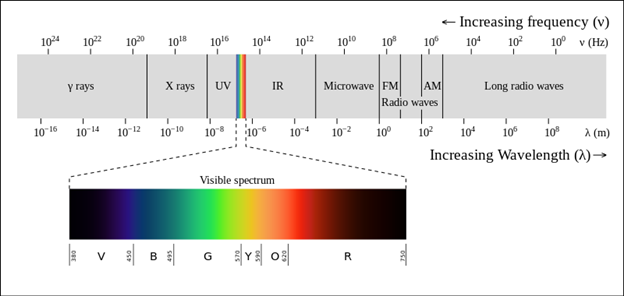
If you know anything about the greenhouse effect (I know very little), you understand that the sun bombards the planet with a spectrum of light: ultraviolet, visible, and infrared, with UV having higher frequencies and IR having lower frequencies. Check out the light spectrum (logarithmic scale) in the first chart below and the sun’s spectrum (linear scale) in the second chart below, with increasing wavelength left to right.
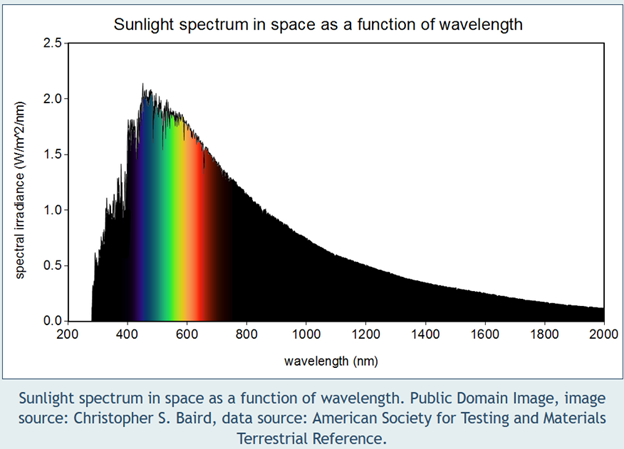 The experts say all reflected light and energy from the planet is longwave infrared. When the frequency, which has an inverse relationship with wavelength, hits a resonate frequency of atmospheric molecules, the molecule absorbs that energy, and there you have the greenhouse effect. Larger molecules have more vibration modes and, therefore, more resonant frequencies (I did remember that from vibrations class); consequently, they absorb more heat. Nitrous oxide, N2O, has a greater global warming potential than methane, CH4. I’m not going to answer everything. Well… I think it’s because nitrous oxide stays in the atmosphere much longer than methane does.
The experts say all reflected light and energy from the planet is longwave infrared. When the frequency, which has an inverse relationship with wavelength, hits a resonate frequency of atmospheric molecules, the molecule absorbs that energy, and there you have the greenhouse effect. Larger molecules have more vibration modes and, therefore, more resonant frequencies (I did remember that from vibrations class); consequently, they absorb more heat. Nitrous oxide, N2O, has a greater global warming potential than methane, CH4. I’m not going to answer everything. Well… I think it’s because nitrous oxide stays in the atmosphere much longer than methane does.
 Hydrogen’s GHG Potential
Hydrogen’s GHG Potential
That takes us to the last zinger: Hydrogen is 11X worse than CO2 for the climate. Whiplash! WHAT?
I wrote above that diatomic molecules like H2 cannot resonate with IR radiation from earth back to space, so how is hydrogen a greenhouse gas? Read the headline. It doesn’t say H2 is a GHG.
Nature is better than anything humans can manufacture to sanitize pollution. For example, microbes evolve to devour petroleum when it is consistently available from natural leaks in the ocean floor. This mitigated 90% of the damage caused by the five million barrel Deepwater Horizon disaster in the Gulf of Mexico. Similarly, hydroxyl radicals in the atmosphere clean up pollutants like methane. However, diatomic hydrogen is an easier food for hydroxyls to digest, and therefore, hydrogen burns up methane-eating hydroxyls, extending the life of the potent GHG, methane.
Dazzle the kids, friends, neighbors, and partners with that.
[1] https://www.energy.gov/eere/fuelcells/hydrogen-production-natural-gas-reforming
[2] https://chemeng-processing.blogspot.com/2010/05/hydrogen-production-by-steam-reforming.html
[3] https://www.eia.gov/environment/emissions/co2_vol_mass.php
[4] https://www.engineeringtoolbox.com/fuels-higher-calorific-values-d_169.html
[5] https://www.eia.gov/energyexplained/units-and-calculators/british-thermal-units.php

 [3]
[3]