Aside from efficiency being a core component of my thermos-fluids courses in engineering school, phase changes were also captivating to me. Phase changes from solid (ice) to liquid (water) to vapor (steam) have been used for hundreds of years, and more recently, the last 150 years, give or take, to generate electricity, refrigerate, freeze and keep us cool in the summer heat. Like water, practically anything will freeze, melt or vaporize. Take copper, please. It is widely used to conduct electricity in homes and buildings. It too melts and vaporizes. When it vaporizes, you don’t want to be near it because that would be an explosion known as arc flash. See the image below if you want a reason to stay out of your home electrical panel.
Warning: Real (actual) gruesome results shown in the video.
Here ends today’s PSA.
Phase-Change Brilliance
To learn all about the benefits of boiling (liquid to vapor) and condensing (vapor to liquid), log on to AESP’s 2020 magazine to learn (almost) All about Heat Pumps. Here is a free excerpt:
Nearly all heat pumps and refrigeration cycles use a refrigerant that boils at a low temperature and pressure and condenses at a high pressure and temperature. The production of high pressures and temperatures requires energy input. Figure 1 shows boiling and condensing pressures at typical cooling (indoor) and outdoor temperatures. The primary energy requirement for a vapor-compression cycle is the compression stage, which is driven by natural gas or electricity, as we shall see. The primary input for the absorption cycle is heat.
The key ingredient in refrigeration is a phase change from vapor to liquid and back to vapor – the refrigeration cycle, requiring energy input to make it work. Refer to the above-linked article. The key ingredient for thermal storage is a phase change from liquid to solid and back to liquid. In both cases, there is a relatively enormous capacity to absorb and release heat at a constant temperature, as we shall see below. This is critical for thermal storage because we want products, people, and animals in pristine condition as close to one temperature as possible. Nobody wants to store ‘cool’ by precooling a building to 70°F and letting the temperature drift up to 78°F on a hot afternoon. Ice cream doesn’t like drifting from -10°F to 5°F and back either. That’s how icy, crystally, hairy, aged ice cream is formed in the bachelor’s freezer.
Freezing
Just about anything will freeze (or melt), aka, change phase. It may not be the substance alone that freezes but the water in the substance, e.g., meat, milk, or peas that freezes. Sprain your ankle or burn your hand? A bag of frozen peas is just what you need. Substances used for freezing and cold storage include carbon dioxide (dry ice), water, and brines (saltwater).
For reference, ammonia is the most common industrial refrigerant, particularly in food processing and freezing. It is also used in the absorption cycle with water. Refrigerants 410a and 134a are common for residential and commercial space cooling. Carbon dioxide is gaining ground as a “low side” (low temperature) refrigerant and as a working fluid in emerging heat pump technologies.
It is the phase change that makes these substances so effective and valuable for thermal, and thus, energy storage. The chart below demonstrates this. The value (heat) is measured on the horizontal axis. You can see how a few ounces of ice at 0°C, or 32°F, keep a Manhattan cold for a while.
On the horizontal axis, I’ve added “Value,” and on the vertical axis, I’ve added “Not Value.” The value is energy storage. The not-value is temperature change. You won’t find this anywhere else, but this is the beauty of these phase change materials. They can be adapted to store and shed a lot of thermal energy at precise temperatures. Water freezes and melts at one precise temperature, 32°F, or 0°C, as shown below. Other materials freeze and melt at other precise temperatures.
Why Phase Change Materials are Critical
Why does this matter? First, if we are to use buildings as grid resources, we need to store energy on the cheap. Batteries? See last week’s post for plenty of analysis. They will never compete with thermal storage.
Second, without phase change materials, we’re riding those diagonal lines shown above, and nobody likes that. Who wants to suffer “freezing” in the morning at 72 degrees and then roasting at 80 degrees in the office to shift or trim a couple of kW?
Third, heat is added or extracted with the lowest possible temperature difference, which means efficiency (any mechanical engineer should know this as well as they know how to tie their shoes). This means ice is not an ideal thermal storage medium, except for things we want to keep near the freezing point of water.
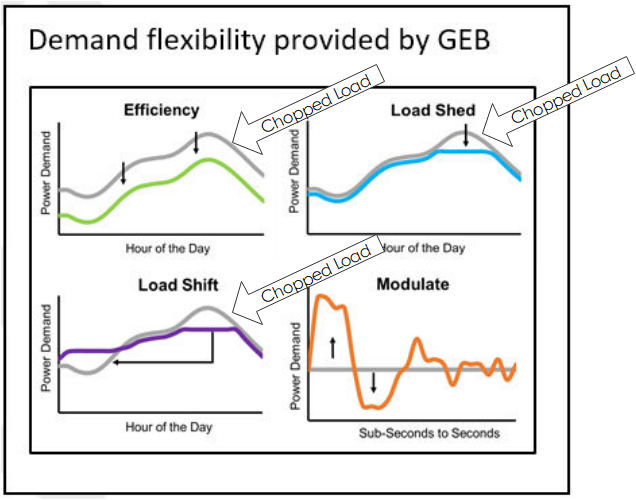
Grid-Interactive Efficient Buildings
Grid-Interactive Efficient Buildings, also known as GEBs, is a concept that is in the incubation phase within the Department of Energy. Grid interactive buildings act like batteries to absorb anything that requires electricity for use at another time, to take peak load off the grid. The image on the right shows crude effects of various strategies to reduce energy costs in buildings while making the grid more resilient. I say they’re crude because efficiency rarely saves ‘X kW’ every hour of the day, as shown.
Efficiency has a load shape like, but not the same as, the load (equipment) it is impacting. Thermal storage may be used to shed or shift load. In both cases, I think you can see how the peak demand is chopped off in the image. The gray curves represent status quo operation and the colored lines are modified using the strategies noted.
We will cover how this can be done next week.
