I took advantage of the cold weather this week to demonstrate ice formation again. Caution: video ahead.
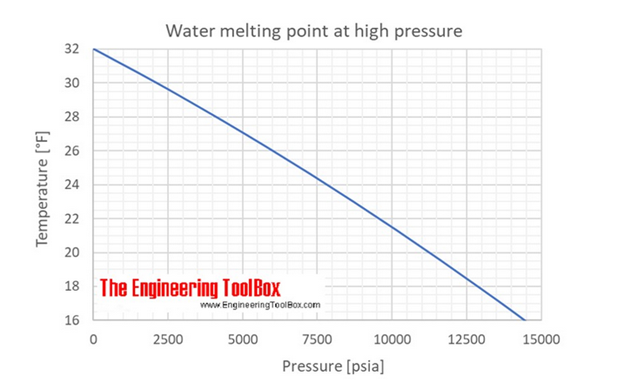
When I take to Google and ask, “What is the freezing point of water?” it apparently depends on my location 🙄. You probably know that the boiling point of water changes with barometric pressure, but the freezing point also changes with pressure – but over a much greater range of pressure, as shown below.
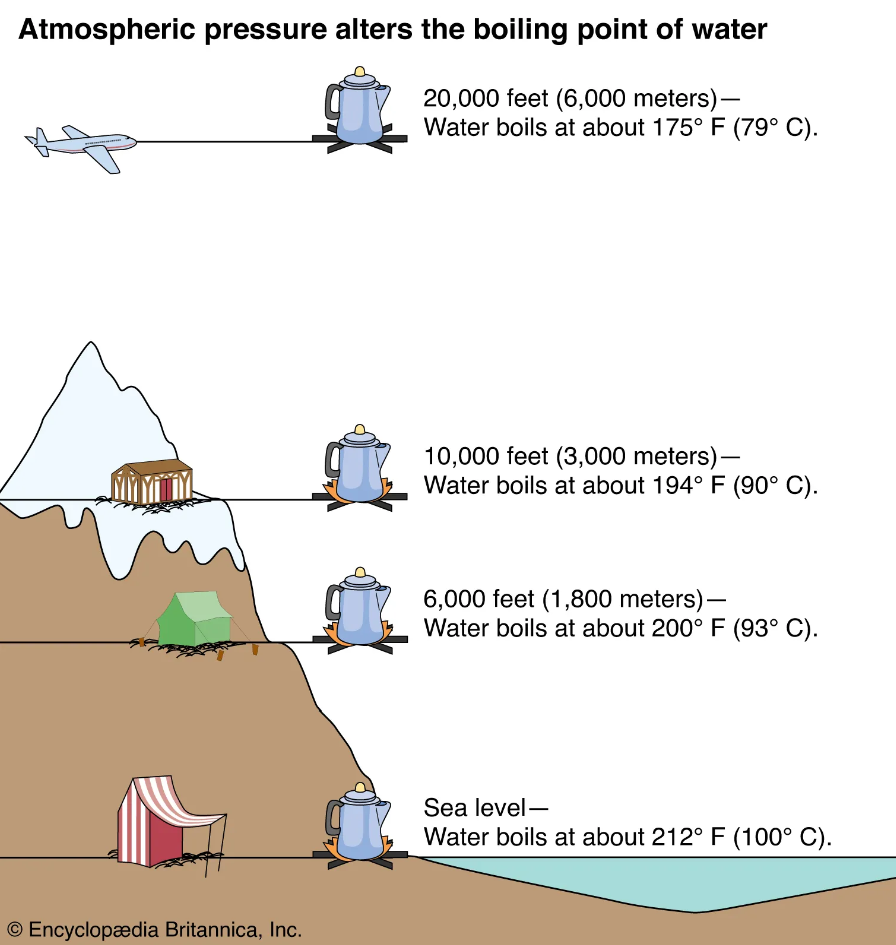 A change in the melting point of ice of two degrees Fahrenheit requires 2000 pounds per square inch (psi). The range of atmospheric pressure everywhere on the planet only varies by about 10 psi. Water’s freeze/melt point changes by only 0.01 degrees anywhere on the planet, so Google doesn’t need my location.
A change in the melting point of ice of two degrees Fahrenheit requires 2000 pounds per square inch (psi). The range of atmospheric pressure everywhere on the planet only varies by about 10 psi. Water’s freeze/melt point changes by only 0.01 degrees anywhere on the planet, so Google doesn’t need my location.
Mysterious Ice
Sometimes, I hit a wall when I research things for the Rant. For instance, I had yet to learn how poorly understood ice is. For example, the rumor I remember from an engineering course is that the pressure of ice skates was significant enough to melt the ice, and that is what makes for easy gliding with almost no friction. That logic doesn’t apply to snow skiing or falling on your keister after slipping on an icy patch of sidewalk. Vox has a good article contemplating the reasons ice is slippery. It might be pressure. No. It might be friction. Could be. It’s a molecular-scale phenomenon at the ice’s surface. Maybe.
The freezing point of water is equally a poorly understood phenomenon. This reputable tutor site says pure water in a beaker can be chilled to minus 40F before it freezes. I would say such a beaker should come straight off the assembly line and transported in a vacuum to a clean room to achieve such a feat. Like snowflake formation, nucleation, the first step in ice crystal formation, is aided by impurities.
Ice Crystals in the Lab and Nature
A week ago, on the anniversary of my first lab experiment with subcooling, I was urged to try it out again. My theory of the case is that cracking the bottle open and flicking it with my finger produces a bubble from dissolved gas in the water, which triggers nucleation. Call it a catalyst to launch the crystallization.
I remember from engineering school, namely mechanics of materials, that the phase change from liquid to solid forms a crystalline structure rather than a solid blob. Crystals grow linearly because when liquid changes phase to solid, it releases heat, just as melting solids absorb heat. The crystal grows in straight lines toward colder liquid. You can see this unfold in the video above and photos below. The dissolved gas starts the process at the top, where I provide disturbance, and the crystals grow from there down.
Aside from the video, we can see crystalline freezing on the sidewalks in the hood last Sunday. Amazing stuff abounds if we slow down and look a little. This first one shows giant crystals that are inches long. Debris along the edges create nucleation sites for ice formation to begin.


The second pic shows shorter crystals. Crystal growth occurs where the water was 100% liquid the day before. The blobs (upper right corner) were not fully melted (slush) the day before. If we had a super-magnifying glass, we could see crystals within the blobs.
The last formation I looked at is shown in the following two photos. The second is a zoom-in of the first. The shorter crystals indicate this ice formed more quickly because the water depth is less than the others. My iPhone thinks the second image is one of a reptile—more flawless artificial intelligence.

 Why is this important?
Why is this important?
The latent heat of fusion (melting or freezing) is the magical phenomenon behind thermal energy storage: the ability to store “cold” and absorb many units of heat[1] with zero change in temperature.
Phase Change Storage Capacity
A gram of water absorbs or releases one calorie of energy per degree C change in temperature. For reference, there are 500 grams in a typical plastic bottle of water. A gram of water releases 80 calories with zero change in temperature when it freezes. In other words, the thermal energy required to melt a gram of ice could heat water from just above freezing to near the boiling point (80 degrees Celsius).
Subcooling Matters
In the Navy, I coined the phrase, “It’s easy if you know how it works.” That was in response to asking for help figuring something out, and the response was, “That’s easy.” That’s thermal storage – it’s easy if you know how it works. If you don’t, look out!
The refrigeration system, including a chilled water plant, must reach a temperature that will catalyze the freezing process. You can see via the video that once the freeze is triggered, the solution immediately regresses to the theoretical freeze point of 32F for water. The refrigeration system doesn’t move all the Btus (or calories) at the subcooling temperature – It only moves the percentage of Btus/calories shown in the following chart. The rest happens at 32F.
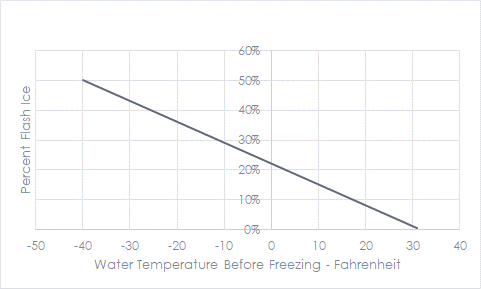 Per the experiment, bottled water will freeze around 20-25F. We raise the freezing point by adding nucleating agents, like the debris on the side of the sidewalk. Any sharp particle or surface projection can act as a nucleating agent.
Per the experiment, bottled water will freeze around 20-25F. We raise the freezing point by adding nucleating agents, like the debris on the side of the sidewalk. Any sharp particle or surface projection can act as a nucleating agent.
There you are. Dazzle the kids.
[1] E.g., British thermal units, calories, Joules, Newton-meters, kWh, ergs, ft-lbf

 Why is this important?
Why is this important?