Last week, we explored ethanol carbon content, carbon caps and markets, and refiner profits on gasoline and diesel fuel. Wasn’t it fantastic stuff? This week, we describe precisely how the fluids you use to fuel and lubricate your vehicle are manufactured, what drives their costs, and how they impact efficiency. Feeling tingly? Let’s go!
Diverging Gasoline and Diesel Costs – Why?
Referring back to the article I mentioned last week from The Wall Street Journal: Gas Prices are Falling, but Refiners Keep Making More, believe me when I say, you are getting more analysis and insight from this blog than is provided by The Wall Street Journal. The crux of that article is that gasoline prices are cratering while what the Journal calls distillates, diesel fuel, and heating oil, prices are high. Why? The gluttonous data mongrel digs in.
The chart features monthly retail gasoline[1] and diesel[2] fuel prices back to December 1994. Prices shown are nominal, which simply means they are not adjusted for inflation. When I look at the price charts, I think, hmm that’s interesting. What does the difference look like? That difference is plotted in the gray curve shown. There are three takeaways here:

- The difference in the cost for diesel fuel (distillate) and gasoline rises in winter for the heating season (heating fuel oil and diesel fuel are close alternatives – more below)
- The price difference picked up substantially starting around 2007. The reason: the EPA lowered the diesel fuel sulfur limit to 15 ppm from 500 ppm for road use and for all diesel sales in 2010.
- Prices have plummeted in the past few years. The reason: domestic production is up a lot.
The Journal article implies that gasoline is practically a coproduct of making distillates – diesel, heating oil, and jet fuel. This is true, especially in the non-summer months. Refer to the Journal’s refiner profit margin chart from last week here. Summer gasoline requires a lower vapor pressure, and that means “heavier” gasoline and probably less ethanol. Less ethanol means less competition for pricing, higher gasoline prices, and higher margins.
Another reason gasoline is a coproduct of making profitable fuel oil is refinery configurations are capable of swinging only 6-8% of their production between gasoline and diesel fuel.
Petrol Product Manufacturing Magic, or Not
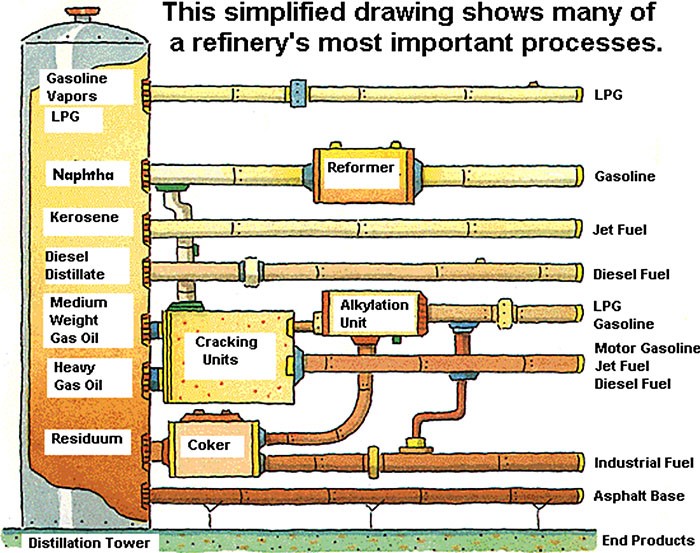
Let us examine how fuels, waxes, and other things are made from crude oil. To an engineer, I think the fuel-making process should be quite easy to understand. Different fluids, from refrigerant to molten copper, and everything in between (liquid fuels) have different boiling temperatures. As such, the refining process is quite simple. Boil crude oil and condense the various fuels at their condensing/boiling temperature. This is called fractionation, which is depicted in the cartoon below[3].

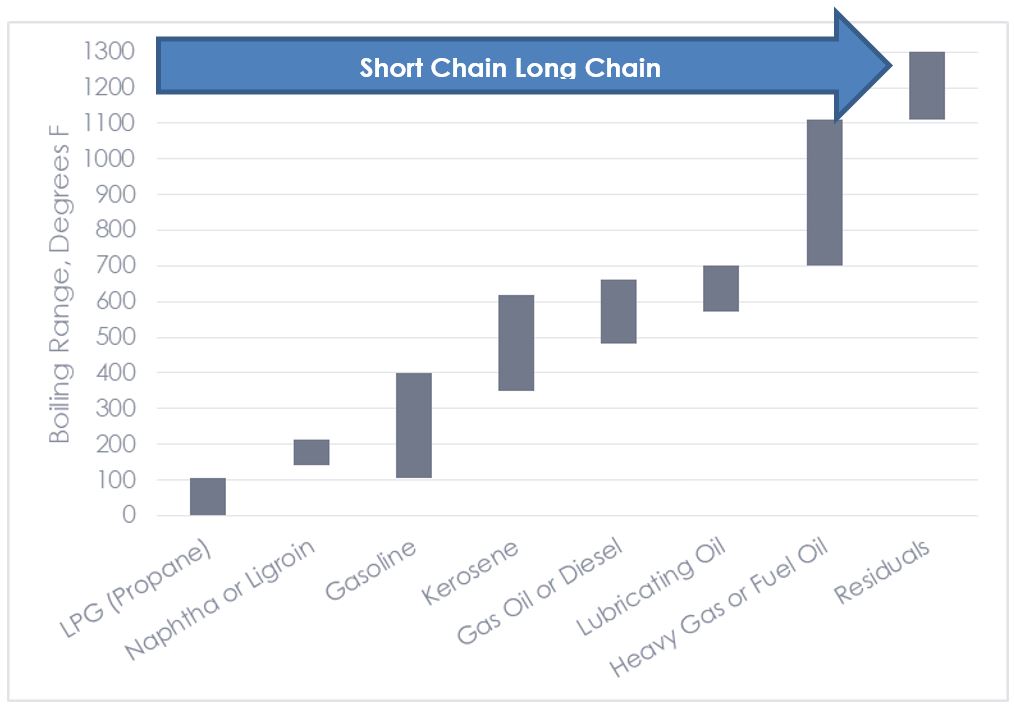
In basic terms, crude oil includes a soup of hydrocarbons, which are simply a wide range of molecules of carbon and hydrogen atoms. The more complex and larger the molecule, the thicker and heavier it is. For example, gaseous[4] molecules include methane, propane, butane, and ethane. From these smaller molecules to larger molecules (thicker stews): gasoline, kerosene, diesel fuel, fuel oil, coke, asphalt, tar, and wax.
The boiling/condensing temperatures of the various products are as shown in the following chart.
Chain Changing
Chemistry wizards have developed ways to convert long-chain molecules (thick stuff) into shorter chain hydrocarbons via “cracking”. A cracking unit is shown in the cartoon above.
The opposite of cracking is unification. Who’d have thunk? This process deploys a catalyst like platinum to convert shorter chain hydrocarbons into longer chain hydrocarbons.
Finally, there is conversion, where the wizards blend bi-product acids to catalyze processes that make hi-value products like octane, which helps avoid engine knock – the simultaneous combustion (explosion) of compressed air/fuel in the engine cylinder. Engine knock is bad because as noted, explosions are hard on engine parts, and they reduce mileage because less work is extracted from the fuel. Did you know the octane rating (87,88, 92, etc.) is the percent octane in gasoline? Octane is an eight-carbon molecule – which begs the question, why is October the tenth month? Discuss.
Sulfurous Maritime Free Rider Days Are Over
Sulfur limits in diesel fuels were drastically reduced in 2007 as noted above. However, maritime shipping, which uses just as much fuel as heavy trucking, was not caught in the regulation. They continue to burn fuel containing about 3.5% sulfur. That is about 2,000 times the sulfur allowed in trucking. Does this make sense? No. Therefore, shippers will be required to either use low sulfur fuel or retrofit with scrubbers, as owners of coal-fired power plants have been required to do. The fuel price increase is expected to be about 55%. It has refiners scrambling to retrofit their processes.
Fuel Types and Fuel Efficiency
There isn’t sufficient space in this post to discuss the all-important fuel efficiency. We will slot that for a future post. Cheers!
[1] https://www.eia.gov/dnav/pet/hist/LeafHandler.ashx?n=PET&s=EMM_EPM0U_PTE_NUS_DPG&f=M
[2] https://www.eia.gov/dnav/pet/hist/LeafHandler.ashx?n=pet&s=emd_epd2d_pte_nus_dpg&f=m
[3] Stolen from Penn State University who reportedly stole it from the EIA, therefore it’s actually ours to use.
[4] Depending on the pressure applied.