
Exergy is a sexy word (maybe only to energy nerds), but it represents a vital class of measures and opportunities as we sprint head-on into the game changer (cliché alert) that will be the post-gravy-train-of-lighting-retrofit-era of energy efficiency. Pay attention!
Exergy, also known as availability, is the total useful energy that can be captured before a system comes into equilibrium with the surroundings. Equilibrium can be as simple as a mass coming to rest.
Potential Energy
Consider a hydropower plant. The potential energy is in the form of elevated water on one side, the reservoir, and a shallow stream for whitewater rafting on the other. The dam shown to the left is the Robert Moses Niagara Power Plant. You can see the water exiting the plant still has exergy in the form of high velocity churning in the river below until it comes into equilibrium with the river’s flow. That is wasted energy.
Temperature
Any thermal engine – in your car or lawnmower, power plant, jet engine, and others — is driven by temperature difference. Any mechanical engineer who knows anything knows the isentropic (reversible) efficiency of an engine’s cycle is proportional to the actual work output divided by the potential output. This is expressed via the Carnot (pronounced car-no) power cycle. The work from the isentropic engine is represented by the temperature difference of the high and low heat reservoirs. The total available energy is represented such that we could suck all the energy from the high temperature to absolute zero, resulting in the following nifty equation that anyone can understand.
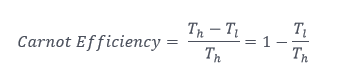
Where the h and l subscripts represent high and low temperatures, respectively.
Power Plants
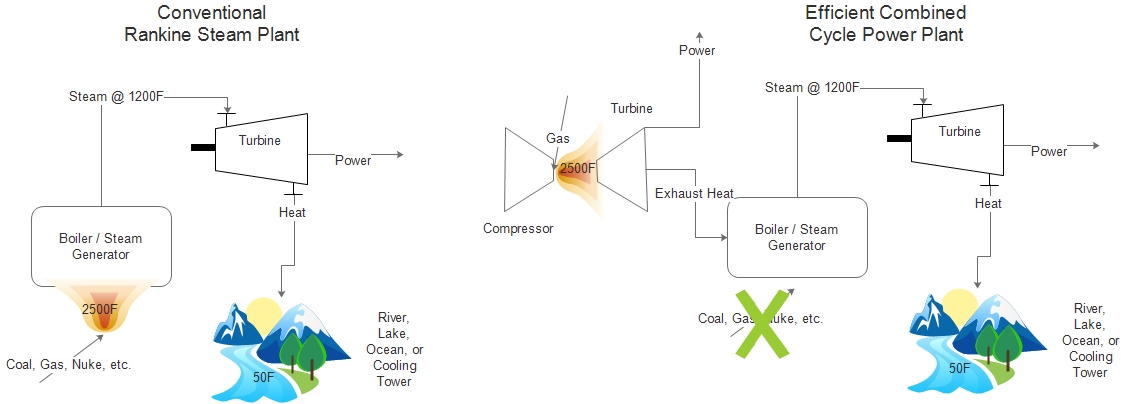
What does it look like for power plants? The concept applies, but since conventional power plants use steam with a phase change from water to steam and back to water, Carnot efficiency does not directly apply. However, combined cycle plants are more efficient because Th is much higher, as demonstrated by Carnot efficiency.
A thermal power plant using steam from nuclear, coal, or natural gas typically has a high temperature of only about 1,200 degrees F within the thermal cycle. The efficiency of such a plant is about 30-35%, depending on energy efficiency features I may discuss later. Most of the 70% loss is heat dumped into the environment (river, lake, ocean, air).
Combined cycle natural gas power plants consist of two heat engines in series: a gas turbine and a conventional thermal power plant, which is also known as the bottoming cycle of the cogeneration plant (another name for the same thing). The high temperature in this plant can be 2,500 degrees F entering the gas turbine. With this higher temperature, the efficiency is almost double the simple thermal plant, even with the same fuel burned at the same temperature.
Temperatures in the simple steam plant are limited by the strength of the tons of carbon steel needed for the plant. For the gas turbine, only a relatively tiny amount of expensive exotic alloys are needed to withstand high temperature.
Therefore, in the case of power plants, more exergy is captured at the high end in a combined cycle plant. This results in greater efficiency. Refer to the cartoons below. The combined cycle plant begins extracting power at 2,500F whereas the conventional steam plant begins extraction at a measly 1,200F. This makes a huge difference in efficiency.
Buildings
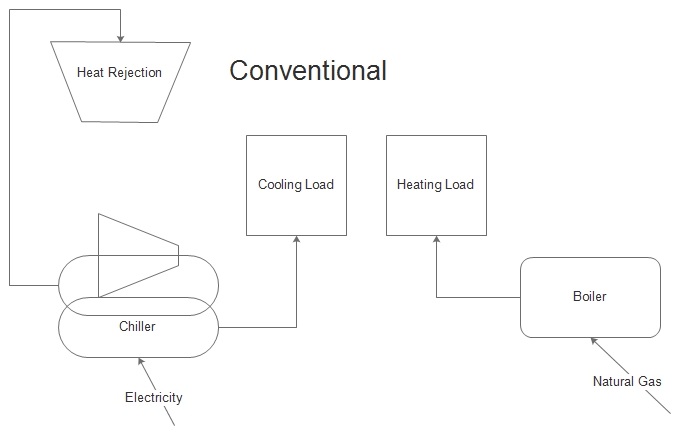
In commercial buildings, and especially industrial buildings, energy is consumed for cooling while at the same time energy is consumed for heating. Heating may be required for space conditioning, water heating, cooking, and many other processes.
Consider a chiller used for air conditioning or industrial process cooling. The norm is to dump the heat outdoors through a cooling tower or an air-cooled condenser. This is lost exergy. At the same time, natural gas is used to heat water for a swimming pool, laundry, showers, or process water heating.
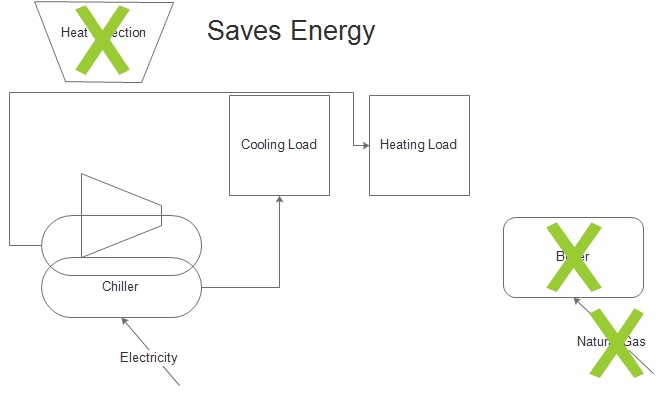
The solution may be a heat recovery chiller. Such a machine will reject heat directly to these other heating loads, avoiding the need to burn natural gas.
I think you can see what I’m saying in the cartoons to the left. The first is the status quo, consuming electricity for cooling and natural gas for heating. The second provides the heating with the heat rejected from the cooling process. It is possible to avoid natural gas use altogether in certain situations; certain being the key word.
Key account managers for large industrial customers, take note. The example below is a perfect reason why industrial customers should not be opted out of efficiency programs. In most cases, if they need more cooling or system replacement, they will add a chiller and a tower. The same thing will happen for heating. Wait! Think different!
One key to exergy is minimize what is thrown away, and that is usually heat. But it also applies to efficiency in a more conventional sense. I will get into that next week.
I love these subjects that are so vast. I can use them for several posts. Next week(s), I will write about Kool Aid drinkers, snake oil salesmen, huge chasms, and itty bitty increments.




