Does anyone make ice cream at home these days? That was one of our favorite treats as kids – back when we had three network channels and no recording devices. I’m not going to look into modern ice cream makers for home, but I’ll bet they have cheaters that don’t require ice and salt. Why is salt used with ice to freeze ice cream? Read on to find out.
 Anyway, I found an early-patented “refrigeration system” (1793) that used ice and salt with charcoal and blankets for insulation. The simple mechanical refrigeration cycle was invented in the early 1900s.
Anyway, I found an early-patented “refrigeration system” (1793) that used ice and salt with charcoal and blankets for insulation. The simple mechanical refrigeration cycle was invented in the early 1900s.
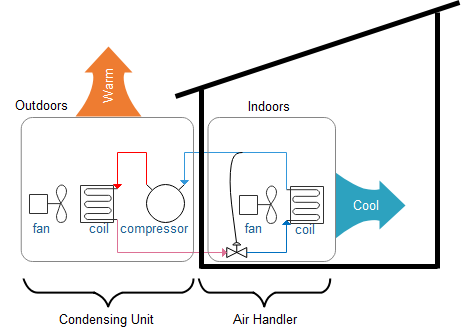
Mechanical Refrigeration Basics
The following cartoon, which may look familiar, is my depiction of a refrigeration system used for space cooling. There are four steps in the cycle. The compressor takes cool, low-pressure gaseous refrigerant and compresses it to high temperature. Substances condense at higher temperatures (outdoors) under high pressure. That is the second step: condensing to liquid refrigerant. Compressor pressure moves the liquid refrigerant back inside the building, where its pressure is decreased across an expansion valve (step three). You guessed it, the liquid at low pressure boils at low temperature, absorbing heat (cooling) in step four of the cycle. Round and round it goes. You just need to add motive energy, usually electricity, to run the compressor.
Back to the ice cream trivia: Adding salt to water (solid or liquid) lowers its freezing point. Therefore, mixing salt with ice drops the ice below freezing (32F or 0C) to speed up ice cream making.
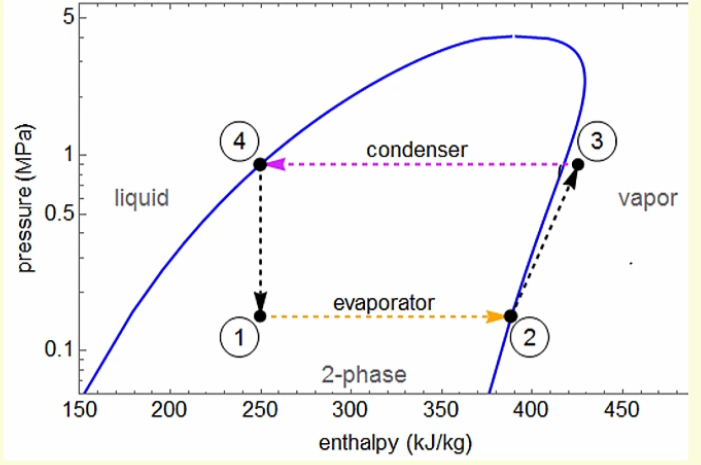
Here is the system on a pressure enthalpy chart. Enthalpy measures the relative heat (energy) in the refrigerant. As it evaporates, it absorbs energy (heat) from the space.
Refrigerants
What is the substance or refrigerant used in the refrigeration system to get to the heart of this post? Anything that boils and condenses can be used as a refrigerant to move heat from a cold body to a warm body. Copper vaporizes and condenses – that could be used. Except that serves no purpose because the temperatures are astronomical. Water is the refrigerant in absorption cycles, but we’re not getting into that complexity today.
Most refrigerants have some, but not all, of these negative characteristics: toxic, flammable, explosive, and corrosive. Some are absent these but come with other baggage – like carbon dioxide. Carbon dioxide’s saturation (boiling/evaporation) pressures are very high, but it can work great in a more complex refrigeration system serving a very cold load, like for freezing food.
Some refrigerants are fine in their pure form, but when they are in the presence of water vapor or some other phenomenon (burned), they may become very corrosive. For example, years ago, I visited a convention facility that was having problems with rapidly failing water heaters. The heat exchangers were failing. They were in the same room as the chiller under which there was oil on the floor. Uh oh. Refrigeration leak. The water heaters were “burning” refrigerant to produce highly corrosive chemicals. Oh, that’s nasty!
Pariah Refrigerants
After floundering with “poor” refrigerants like water, CO2, ammonia, and ethers, the chlorofluorocarbon (CFC) series were introduced. The Kleenex, Q-Tip, Band-Aid name for them was Freon. These refrigerants were indestructible (except when exposed to UV light) and didn’t react with anything (except if burned – there is always a catch). In the 70s, it was discovered these refrigerants, when in the stratosphere, break down in UV light from the sun and the chlorine “drifts free[1].” The free chlorine atoms convey elemental oxygen that rob an O from O3 (ozone) to create stable O2 we love and breathe[2].
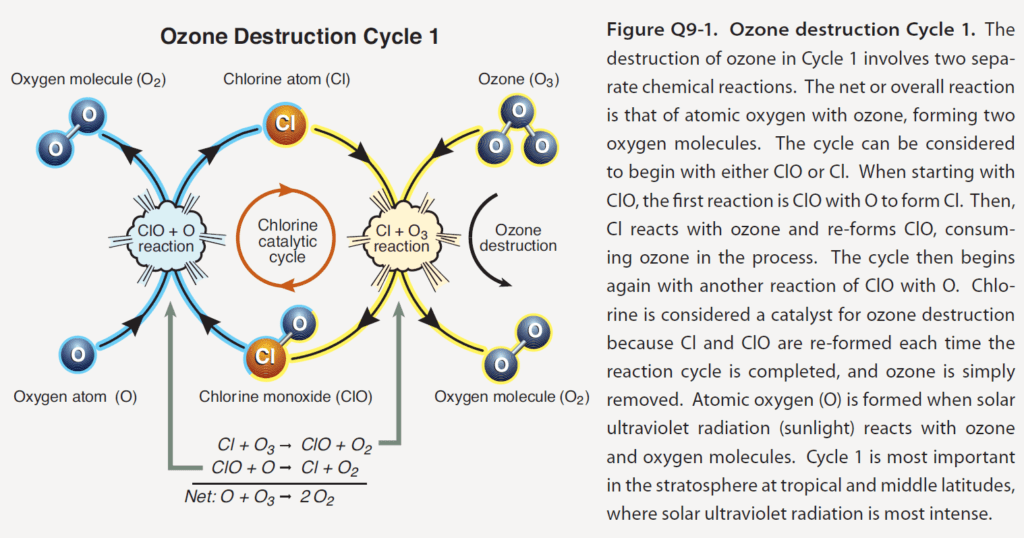
The CFCs were phased out and replaced with HCFCs, where the H is hydrogen, hydrochlorofluorocarbon. Later HFCs, hydrofluorocarbons, were developed.
Some engineers believed the CFC ozone depletion was a scam because the CFC molecules are much denser than air, so how do they get whipped up to the stratosphere where they are shattered to bits? The scam would have been driven by patent expiration, so manufacturers needed an excuse to make new patented refrigerants. True?
I learned the ozone hole only exists when it’s cold and pitch black over Antarctica. So, ozone, which is required to protect us from UV light, is created by the sun. The hole disappears when the sun is out, and the hole reappears just in time for hanging out with the penguins in -100F darkness. This is new news to me. Discuss.
Natural Refrigerants
As usual, simplicity is best, except there’s little money in that. After 100 years of screwing around with giant molecules for refrigerants, we are back to the simplicity of “natural” refrigerants: water, hydrocarbons (you got that right), CO2, and NH3 (ammonia). Did you know that ammonia has zero global warming potential? I did not. In fact, my understanding was that any molecule consisting of more than two atoms is a greenhouse gas. The only answer I could find for ammonia not being a GHG is that it disintegrates almost immediately. Ammonia is also a great potential transportation fuel. Do you see any carbon in NH3? I do not.
Burning ammonia:
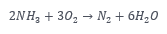
Why are natural refrigerants not the dominant refrigerants? Probably for the same reason efficiency isn’t the dominant energy resource. They are simple, plentiful, safe, and inexpensive, and as a result, have no lobbyists or press. If it bleeds, it leads. If it’s cheap, smart, and safe, the people that normally tell us what to think are mute.
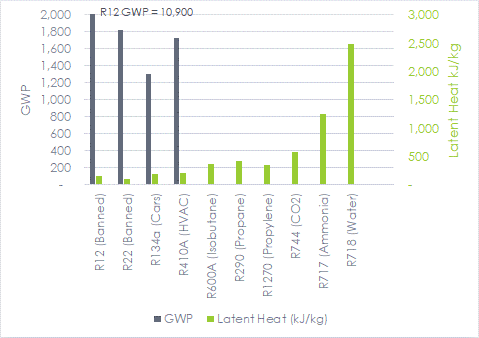
Look at the global warming potential (GWP) in the chart of refrigerant qualities below[3][4], where carbon dioxide is the base, 1.0. The HVAC refrigerant of choice, R410a, is about 1700x the greenhouse gas compared to CO2. The natural refrigerants have unnoticeable GWP and much higher latent heat content, which is good! So, why not natural refrigerants?